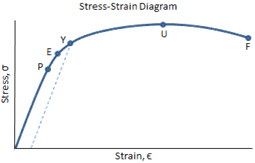
Engineering Materials
Engineering materials refers to the group of materials that are used in the construction of manmade structures and components. The primary function of an engineering material is to withstand applied loading without breaking and without exhibiting excessive deflection. The major classifications of engineering materials include metals, polymers, ceramics, and composites. The important characteristics of the materials within each of these classes are discussed on this page, and tables of material properties are also provided.
Contents
Metals
Metals are the most commonly used class of engineering material. Metal alloys are especially common, and they are formed by combining a metal with one or more other metallic and/or non-metallic materials. The combination usually occurs through a process of melting, mixing, and cooling. The goal of alloying is to improve the properties of the base material in some desirable way.
Metal alloy compositions are described in terms of the percentages of the various elements in the alloy, where the percentages are measured by weight.
Ferrous Alloys
Ferrous alloys have iron as the base element. These alloys and include steels and cast irons. Ferrous alloys are the most common metal alloys in use due to the abundance of iron, ease of production, and high versatility of the material. The biggest disadvantage of many ferrous alloys is low corrosion resistance.
Carbon is an important alloying element in all ferrous alloys. In general, higher levels of carbon increase strength and hardness, and decrease ductility and weldability.
Carbon Steel
Carbon steels are basically just mixtures of iron and carbon. They may contain small amounts of other elements, but carbon is the primary alloying ingredient. The effect of adding carbon is an increase in strength and hardness.
Most carbon steels are plain carbon steels, of which there are several types.
Low-Carbon Steel
Low-carbon steel has less than about 0.30% carbon. It is characterized by low strength but high ductility. Some strengthening can be achieved through cold working, but it does not respond well to heat treatment. Low-carbon steel is very weldable and is inexpensive to produce. Common uses for low-carbon steel include wire, structural shapes, machine parts, and sheet metal.
Medium-Carbon Steel
Medium-carbon steel contains between about 0.30% to 0.70% carbon. It can be heat treated to increase strength, especially with the higher carbon contents. Medium-carbon steel is frequently used for axles, gears, shafts, and machine parts.
High-Carbon Steel
High-carbon steel contains between about 0.70% to 1.40% carbon. It has high strength but low ductility. Common uses include drills, cutting tools, knives, and springs.
Carbon Steel Materials Table
The table below provides representative mechanical properties for several common carbon steels. (Note 1)
| Material | Condition | Yield Strength [ksi] |
Ultimate Strength [ksi] |
Elongation % |
Elastic Modulus [psi] |
Density [lb/in3] |
Poisson's Ratio |
|---|---|---|---|---|---|---|---|
| AISI 1020 | Hot Rolled | 32 | 50 | 25 | 29e6 | 0.283 | 0.32 |
| Cold Worked | 60 | 70 | 5 | ||||
| Stress Relieved | 50 | 65 | 10 | ||||
| Annealed | 28 | 48 | 30 | ||||
| Normalized | 34 | 55 | 22 | ||||
| AISI 1045 | Hot Rolled | 45 | 75 | 15 | 29e6 | 0.283 | 0.32 |
| Cold Worked | 80 | 90 | 5 | ||||
| Stress Relieved | 70 | 80 | 8 | ||||
| Annealed | 35 | 65 | 20 | ||||
| Normalized | 48 | 75 | 15 | ||||
| ASTM A36 | 36 | 58 | 21 | 29e6 | 0.283 | 0.3 | |
| ASTM A516 | Grade 70 | 38 | 70 | 17 | 29e6 | 0.283 | 0.3 |
| NOTE : See our materials database for data conforming to specific material specifications. | |||||||
Low-Alloy Steel
Low-alloy steels, also commonly called alloy steels, contain less than about 8% total alloying ingredients. Low-alloy steels are typically stronger than carbon steels and have better corrosion resistance.
Some low-alloy steels are designated as high-strength low-alloy (HSLA) steels. What sets HSLA steels apart from other low-alloy steels is that they are designed to achieve specific mechanical properties rather than to meet a specific chemical composition.
The table below provides representative mechanical properties for several common alloy steels. (Note 1)
| Material | Condition | Yield Strength [ksi] |
Ultimate Strength [ksi] |
Elongation % |
Elastic Modulus [psi] |
Density [lb/in3] |
Poisson's Ratio |
|---|---|---|---|---|---|---|---|
| AISI 4130 | Hot Rolled | 70 | 90 | 20 | 29e6 | 0.283 | 0.32 |
| Stress Relieved | 85 | 105 | 10 | ||||
| Annealed | 55 | 75 | 30 | ||||
| Normalized | 60 | 90 | 20 | ||||
| AISI 4140 | Hot Rolled | 90 | 120 | 15 | 29.7e6 | 0.283 | 0.32 |
| Stress Relieved | 100 | 120 | 10 | ||||
| Annealed | 60 | 80 | 25 | ||||
| Normalized | 90 | 120 | 20 | ||||
| ASTM A242 | 46 | 67 | 18 | 30e6 | 0.282 | 0.3 | |
| ASTM A302 | Grade A | 45 | 75 | 15 | 29e6 | 0.282 | 0.29 |
| Grade C | 50 | 80 | 17 | ||||
| ASTM A514 | Quenched & Tempered | 100 | 110 | 18 | 29e6 | 0.283 | 0.3 |
| ASTM A517 | Grade F | 100 | 115 | 16 | 29e6 | 0.280 | 0.29 |
| ASTM A533 | Class 1 | 50 | 80 | 18 | 29e6 | 0.282 | 0.29 |
| Class 2 | 70 | 90 | 16 | ||||
| Class 3 | 83 | 100 | 16 | ||||
| ASTM A572 | Grade 50 | 50 | 65 | 18 | 30e6 | 0.283 | 0.3 |
| ASTM A588 | 50 | 70 | 18 | 29.7e6 | 0.280 | 0.28 | |
| ASTM A633 | Grade E | 55 | 75 | 18 | 29.7e6 | 0.280 | 0.28 |
| ASTM A656 | Grade 50 | 50 | 60 | 20 | 29e6 | 0.282 | 0.29 |
| Grade 60 | 60 | 70 | 17 | ||||
| Grade 70 | 70 | 80 | 14 | ||||
| Grade 80 | 80 | 90 | 12 | ||||
| Grade 100 | 100 | 110 | 12 | ||||
| ASTM A710 | Grade A | 80 | 85 | 20 | 29.7e6 | 0.280 | 0.3 |
| HY-80 | 80 | --- | 18 | 29.7e6 | 0.280 | 0.3 | |
| HY-100 | 100 | --- | 16 | 29.7e6 | 0.284 | 0.3 | |
| NOTE : See our materials database for data conforming to specific material specifications. | |||||||
Tool Steel
Tool steels are primarily used to make tooling for use in manufacturing, for example cutting tools, drill bits, punches, dies, and chisels. Alloying elements are typically chosen to optimize hardness, wear resistance, and toughness.
Stainless Steel
Stainless steels have good corrosion resistance, mostly due to the addition of chromium as an alloying ingredient. Stainless steels have a chromium composition of at least 11%. Passivation occurs with chromium content at or above 12%, in which case a protective inert film of chromic oxide forms over the material and prevents oxidation. The corrosion resistance of stainless steel is a result of this passivation.
The table below shows the typical compositions of stainless steels:
| Element | Austenitic | Ferritic | Martensitic |
|---|---|---|---|
| Carbon | 0.03 - 0.25% | 0.08 - 0.20% | 0.15 - 1.2% |
| Chromium | 16 - 26% | 11 - 27% | 11.5 - 18% |
| Nickel | 3.5 - 22% | --- | --- |
| Manganese | 2% | 1 - 1.5% | 1% |
| Silicon | 1 - 2% | 1% | 1% |
| NOTE : Table adapted from Lindeburg. | |||
Austenitic Stainless Steel
Austenitic stainless steel is the most common form of stainless steel. It has the highest general corrosion resistance among stainless steels. It is also the most weldable of the stainless steels due to its low carbon content. It can only be strengthened through cold work. Austenitic stainless steels are generally more expensive than other stainless steels due to nickel content. Austenitic stainless steels are not magnetic, although ferritic and martensitic stainless steels are. Common applications include fasteners, pressure vessels, and piping.
Ferritic Stainless Steel
Ferritic stainless steel has high chromium content and medium carbon content. It has good corrosion resistance rather than high strength. It generally cannot be strengthened through heat treatment, and can only be strengthened via cold work.
Martensitic Stainless Steel
Martensitic stainless steel has high carbon content (up to 2%) and low chromium content. This higher carbon content is the primary difference between ferritic and martensitic stainless steels. Due to the high carbon content, it is difficult to weld. It can be strengthened through heat treatment. Common applications include cutlery and surgical instruments.
Duplex Stainless Steel
Duplex stainless steel contains both austenitic and ferritic phases. It can have up to twice the strength of austenitic stainless steel. It also has a high toughness, corrosion resistance, and wear resistance. Duplex stainless steel is generally as weldable as austenitic, but it has a temperature limit.
Precipitation-Hardenable Stainless Steel
Precipitation-hardenable stainless steel can be strengthened through precipitation hardening, which is an age hardening process. These materials have high strength as well as high resistance to corrosion and temperature.
Stainless Steel Materials Table
The table below provides representative mechanical properties for several common stainless steels. (Note 1)
| Material | Class | Condition | Yield Strength [ksi] |
Ultimate Strength [ksi] |
Elongation % |
Elastic Modulus [psi] |
Density [lb/in3] |
Poisson's Ratio |
|---|---|---|---|---|---|---|---|---|
| AISI 201 | Austenitic | Annealed | 40 | 75 | 40 | 28e6 | 0.289 | 0.27 |
| AISI 202 | Austenitic | Annealed | 40 | 75 | 40 | 28e6 | 0.289 | 0.27 |
| AISI 302 | Austenitic | Annealed | 30 | 75 | 40 | 28e6 | 0.289 | 0.27 |
| AISI 304 | Austenitic | Annealed | 30 | 75 | 40 | 28e6 | 0.289 | 0.29 |
| AISI 304L | Austenitic | Annealed | 25 | 70 | 40 | 28e6 | 0.289 | 0.28 |
| AISI 316 | Austenitic | Annealed | 30 | 75 | 40 | 28e6 | 0.289 | 0.26 |
| AISI 316L | Austenitic | Annealed | 25 | 70 | 40 | 28e6 | 0.289 | 0.26 |
| AISI 405 | Ferritic | 25 | 60 | 20 | 29e6 | 0.282 | 0.28 | |
| AISI 410 | Martensitic | Annealed | 40 | 70 | 16 | 29e6 | 0.282 | 0.28 |
| Quenched & Tempered | 80 | 100 | 12 | |||||
| AISI 430 | Ferritic | 30 | 60 | 20 | 29e6 | 0.282 | 0.28 | |
| AISI 446 | Ferritic | Annealed | 40 | 65 | 16 | 29e6 | 0.282 | 0.28 |
| 15-5PH | Martensitic precipitation hardenable | H900 | 170 | 190 | 10 | 28.5e6 | 0.283 | 0.27 |
| H1025 | 145 | 155 | 12 | |||||
| H1150 | 105 | 135 | 16 | |||||
| 17-4PH | Martensitic precipitation hardenable | H900 | 170 | 190 | 10 | 28.5e6 | 0.282 | 0.27 |
| H1025 | 145 | 155 | 12 | |||||
| H1150 | 105 | 135 | 16 | |||||
| 17-7PH | Semiaustenitic precipitation hardenable | TH1050 | 150 | 177 | 6 | 29e6 | 0.276 | 0.28 |
| A-286 | Austenitic precipitation hardenable | 95 | 140 | 15 | 29.1e6 | 0.287 | 0.31 | |
| Alloy 2205 | Duplex Austenitic-Ferritic | 65 | 95 | 25 | 28.5e6 | 0.287 | 0.27 | |
| Ferrallium 255 | Duplex Austenitic-Ferritic | 80 | 110 | 15 | 28.5e6 | 0.287 | 0.27 | |
| NOTE : See our materials database for data conforming to specific material specifications. | ||||||||
Cast Iron
Cast iron is a ferrous alloy containing high levels of carbon, generally greater than 2%. The carbon present in the cast iron can take the form of graphite or carbide. Cast irons have a low melting temperature which makes them well suited to casting.
Gray Cast Iron
Gray cast iron is the most common type. The carbon is in the form of graphite flakes. Gray cast iron is a brittle material, and its compressive strength is much higher than its tensile strength. The fracture surface of gray cast iron has a gray color, which is how it got its name.
Ductile Cast Iron (Nodular Cast Iron)
The addition of magnesium to gray cast iron improves the ductility of the material. The resulting material is called nodular cast iron because the magnesium causes the graphite flakes to form into spherical nodules. It is also called ductile cast iron. Nodular cast iron has good strength, ductility, and machinability. Common uses include crankshafts, gears, pump bodies, valves, and machine parts.
White Cast Iron
White cast iron has carbon in the form of carbide, which makes the material hard, brittle, and difficult to machine. White cast iron is primarily used for wear-resisting components as well as for the production of malleable cast iron.
Malleable Cast Iron
Malleable cast iron is produced by heat treating white cast iron. The heat treatment improves the ductility of the material while maintaining its high strength.
Cast Iron Materials Table
The table below provides representative mechanical properties for several common cast irons. (Note 1)
| Material | Class | Condition | Yield Strength [ksi] |
Ultimate Strength [ksi] |
Elongation % |
Elastic Modulus [psi] |
Density [lb/in3] |
Poisson's Ratio |
|---|---|---|---|---|---|---|---|---|
| ASTM A159 | Gray Cast Iron | G1800 | --- | 18 | --- | 9.6 - 14e6 | 0.264 | 0.26 |
| G2500 | --- | 25 | --- | 12 - 15e6 | ||||
| G3000 | --- | 30 | --- | 13 - 16.4e6 | ||||
| G3500 | --- | 35 | --- | 14.5 - 17e6 | ||||
| G4000 | --- | 40 | --- | 16 - 20e6 | ||||
| ASTM A536 | Ductile Cast Iron | Grade 60-40-18 | 40 | 60 | 18 | 24.5e6 | 0.256 | 0.29 |
| Grade 65-45-12 | 45 | 65 | 12 | 24.5e6 | 0.256 | 0.3 | ||
| Grade 80-55-06 | 55 | 80 | 6 | 24.5e6 | 0.256 | 0.31 | ||
| Grade 100-70-03 | 70 | 100 | 3 | 24.5e6 | 0.256 | 0.3 | ||
| Grade 120-90-02 | 90 | 120 | 2 | 23.8e6 | 0.256 | 0.28 | ||
| NOTE : See our materials database for data conforming to specific material specifications. | ||||||||
We have a number of structural calculators to choose from. Here are just a few:
Aluminum Alloys
Pure aluminum is soft and weak, but it can be alloyed to increase strength. Pure aluminum has good corrosion resistance due to an oxide coating that forms over the material and prevents oxidation. Alloying the aluminum tends to reduce its corrosion resistance.
Aluminum is a widely used material, particularly in the aerospace industry, due to its light weight and corrosion resistance. Despite the fact that aluminum alloys are generally not as strong as steels, they nevertheless have a good strength-to-weight ratio.
Aluminum alloys are named according to a 4-digit number, where the first number indicates the major alloying element. A processing code follows the 4-digit number, which indicates the condition and treatment of the material.
| Series | Major Alloying Element | Heat Treatable |
|---|---|---|
| 1XXX | None (commercially pure) | No |
| 2XXX | Copper | Yes |
| 3XXX | Manganese | No |
| 4XXX | Silicon | No (mostly) |
| 5XXX | Magnesium | No |
| 6XXX | Magnesium and Silicon | Yes |
| 7XXX | Zinc | Yes |
| Suffix | Treatment |
|---|---|
| -F | As fabricated |
| -O | Annealed |
| -HX | Cold worked (strain hardened) |
| -TX | Solution heat treated, precipitation hardened |
The 2000, 6000, and 7000 series aluminum alloys can all be heat treated, and therefore these can achieve the highest strengths. The other alloys can be strengthened through cold work.
The table below provides representative mechanical properties for several common aluminum alloys. (Note 1)
| Material | Condition | Yield Strength [ksi] |
Ultimate Strength [ksi] |
Elongation % |
Elastic Modulus [psi] |
Density [lb/in3] |
Poisson's Ratio |
|---|---|---|---|---|---|---|---|
| Al 2014 | T6, T651 | 59 | 67 | 7 | 10.5e6 | 0.101 | 0.33 |
| Al 2024 | T4 | 40 | 62 | 10 | 10.5e6 | 0.1 | 0.33 |
| Al 5052 | H32 | 23 | 38 | 9 | 10.1e6 | 0.097 | 0.33 |
| Al 5083 | H116, H321 | 31 | 44 | 10 | 10.3e6 | 0.096 | 0.33 |
| H32 | 31 | 56 | 12 | ||||
| Al 6061 | T4 | 16 | 26 | 16 | 9.9e6 | 0.098 | 0.33 |
| T6 | 35 | 38 | 8 | ||||
| Al 7075 | T6, T651 | 68 | 78 | 6 | 10.3e6 | 0.101 | 0.33 |
| NOTE : See our materials database for data conforming to specific material specifications. | |||||||
Nickel Alloys
Nickel alloys have high temperature and corrosion resistance. Common alloying ingredients include copper, chromium, and iron. Common nickel alloys include Monel, K-Monel, Inconel, and Hastelloy.
The table below provides representative mechanical properties for several common nickel alloys. (Note 1)
| Material | Condition | Yield Strength [ksi] |
Ultimate Strength [ksi] |
Elongation % |
Elastic Modulus [psi] |
Density [lb/in3] |
Poisson's Ratio |
|---|---|---|---|---|---|---|---|
| Hastelloy C-276 | Solution annealed | 41 | 100 | 40 | 29.8e6 | 0.321 | 0.28 |
| Inconel 625 | Grade 1 | 55 | 110 | 30 | 29.8e6 | 0.305 | 0.28 |
| Grade 2 | 40 | 100 | 30 | ||||
| Inconel 686 | Grade 1 | 85 | 120 | 20 | 29.8e6 | 0.315 | 0.28 |
| Grade 2 | 125 | 135 | 20 | ||||
| Grade 3 | 150 | 160 | 20 | ||||
| Inconel 718 | Solution annealed & aged | 120 | 150 | 20 | 29.4e6 | 0.297 | 0.29 |
| Solution heat treated | 150 | 180 | 10 | ||||
| Inconel 725 | Solution annealed | 40 | 75 | 45 | 29.6e6 | 0.3 | 0.31 |
| Solution annealed & aged | 120 | 150 | 20 | ||||
| Monel 400 | Annealed | 25 | 70 | 35 | 26e6 | 0.319 | 0.32 |
| Hot worked | 40 | 75 | 30 | ||||
| Cold worked, stress relieved | 50 | 80 | 20 | ||||
| Monel K-500 | Annealed & aged | 85 | 130 | 20 | 26e6 | 0.306 | 0.32 |
| Cold worked & aged | 100 | 140 | 15 | ||||
| NOTE : See our materials database for data conforming to specific material specifications. | |||||||
Copper Alloys
Copper alloys are generally characterized as being electrically conductive, having good corrosion resistance, and being relatively easy to form and cast. While they are a useful engineering material, copper alloys are also very attractive and are commonly used in decorative applications.
Copper alloys primarily consist of brasses and bronzes. Zinc is the major alloying ingredient in brass. Tin is a major alloying element in most bronzes. Bronzes may also contain aluminum, nickel, zinc, silicon, and other elements. The bronzes are typically stronger than the brasses while still maintaining good corrosion resistance.
The aluminum bronze alloys are very hard and have good wearing properties, and so are commonly used in bearing applications. The beryllium copper alloys have good strength and fatigue properties, and good wear resistance when lubricated properly. Beryllium copper is commonly used for springs, bearings, and bushings.
The table below provides representative mechanical properties for several common copper alloys. (Note 1)
| Material | Condition | Yield Strength [ksi] |
Ultimate Strength [ksi] |
Elongation % |
Elastic Modulus [psi] |
Density [lb/in3] |
Poisson's Ratio |
|---|---|---|---|---|---|---|---|
| 70/30 Copper-Nickel | Annealed | 18 | 45 | 30 | 21.8e6 | 0.323 | 0.3 |
| Cold worked | 50 | 65 | 10 | ||||
| 90/10 Copper-Nickel | Annealed | 15 | 38 | 30 | 20.3e6 | 0.323 | 0.3 |
| Cold worked | 30 | 50 | 15 | ||||
| Aluminum Bronze | 32 | 85 | 12 | 15.5e6 | 0.269 | 0.316 | |
| Beryllium Copper | Solution heat treated | 75 | 85 | 8 | 18.5e6 | 0.298 | 0.27 |
| Precipitation heat treated | 140 | 165 | 3 | ||||
| Nickel Aluminum Bronze 632 | Annealed | 34 | 90 | 10 | 16.7e6 | 0.274 | 0.32 |
| Quench hardened | 50 | 90 | 15 | ||||
| NOTE : See our materials database for data conforming to specific material specifications. | |||||||
Titanium Alloys
Titanium alloys are light, strong, and have high corrosion resistance. Their density is much lower than steel, and their strength-to-weight ratio is excellent. For this reason, titanium alloys are used fairly commonly, especially in the aerospace industry. One primary downside of titanium alloys is the high cost.
There are three categories of titanium alloys: alpha alloys, beta alloys, and alpha-beta alloys. Alpha alloys do not respond to heat treatment and are instead strengthened through solid-solution strengthening processes. The beta and alpha-beta alloys can be strengthened by heat treatment, primarily through precipitation hardening.
Titanium alloys are identified using the percentages of alloying elements, for example Ti-6Al-4V.
The table below provides representative mechanical properties for several common titanium alloys. (Note 1)
| Material | Condition | Yield Strength [ksi] |
Ultimate Strength [ksi] |
Elongation % |
Elastic Modulus [psi] |
Density [lb/in3] |
Poisson's Ratio |
|---|---|---|---|---|---|---|---|
| Commercially Pure | Grade 2 | 40 | 50 | 20 | 14.8e6 | 0.163 | 0.34 |
| Ti-5Al-2.5Sn | Annealed | 110 | 115 | 10 | 15.5e6 | 0.162 | 0.31 |
| Ti-6Al-4V | Grade 5 | 120 | 130 | 10 | 16e6 | 0.16 | 0.31 |
| Ti-6Al-4V, ELI | Grade 23 | 110 | 120 | 10 | 16.5e6 | 0.16 | 0.31 |
| Ti-5-1-1-1 | Grade 32 | 85 | 100 | 10 | 16e6 | 0.16 | 0.31 |
| NOTE : See our materials database for data conforming to specific material specifications. | |||||||
We have a number of structural calculators to choose from. Here are just a few:
Polymers
Polymers are materials that consist of molecules formed by long chains of repeating units. They may be natural or synthetic. Many useful engineering materials are polymers, such as plastics, rubbers, fibers, adhesives, and coatings. Polymers are classified as thermoplastic polymers, thermosetting polymers (thermosets), and elastomers.
Thermoplastic Polymers
The classification of thermoplastics and thermosets is based on their response to heat. If heat is applied to a thermoplastic, it will soften and melt. Once it is cooled, it will return to solid form. Thermoplastics do not experience any chemical change through repeated heating and cooling (unless the temperature is high enough to break the molecular bonds). They are therefore very well suited to injection molding.
Thermosetting Polymers
Thermosets are typically heated during initial processing, after which they become permanently hard. Thermosets will not melt upon reheating. If the applied heat becomes extreme however, the thermoset will degrade due to breaking of the molecular bonds. Thermosets typically have greater hardness and strength than thermoplastics. They also typically have better dimensional stability than thermoplastics, meaning that they are better at maintaining their original dimensions when subjected to temperature and moisture changes.
Elastomers
Elastomers are highly elastic polymers with mechanical properties similar to rubber. Elastomers are commonly used for seals, adhesives, hoses, belts, and other flexible parts. The strength and stiffness of rubber can be increased through a process called vulcanization, which involves adding sulfur and subjecting the material to high temperature and pressure. This process causes cross-links to form between the polymer chains.
Ceramics
Ceramics are solid compounds that may consist of metallic or nonmetallic elements. The primary classifications of ceramics include glasses, cements, clay products, refractories, and abrasives.
Ceramics generally have excellent corrosion and wear resistance, high melting temperature, high stiffness, and low electrical and thermal conductivity. Ceramics are also very brittle materials.
Glass
Glasses are common materials and are seen in applications including windows, lenses, and containers. Glasses are amorphous, whereas the other ceramics are mainly crystalline. Primary advantages of glasses include transparency and ease of fabrication. The base element of most glasses is silica, and other components can be added to modify its properties. Common processes used to form glass include:
- heating until melting, then pouring into molds to cast into useful shapes
- heating until soft, then rolling
- heating until soft, then blowing into desired shapes
Cements
Cements are materials that, after mixing with water, form a paste that then hardens. Because of this characteristic, cements can be formed into useful shapes while in paste form before they harden into rigid structures. Plaster of paris is one common cement. The most common cement is called Portland cement, which is made by mixing clay and limestone and then firing at high temperature. Portland cement is used to form concrete, which is made by mixing it with sand, gravel, and water. It can also be mixed with sand and water to form mortar. Like other ceramics, cements are weak in tension but strong in compression. Cement is very inexpensive to produce, and it is used widely in the construction of buildings, bridges, and other large structures.
Clay Products
Clay is a very common ceramic material. It can be mixed with water, shaped, and then hardened through firing at high temperature. The two primary classifications of clay products include structural clay products and whitewares. Structural clay products see applications including bricks, tiles, and piping. Whitewares see applications including pottery and plumbing fixtures.
Refractories
Refractory ceramics can withstand high temperatures and extreme environments. They can also provide thermal insulation. Brick is the most common refractory ceramic.
Abrasives
Abrasive ceramics are hard materials that are used to cut, grind, and wear away other softer materials. Typical properties of abrasives include high hardness, wear resistance, and temperature resistance. Abrasives can either be bonded to a surface (e.g., grinding wheels and sandpaper), or can be used as loose grains (e.g., sand blasting). Common abrasives include cemented carbide, silicon carbide, tungsten carbide, aluminum oxide, and silica sand. Diamond is also an excellent abrasive, but it is expensive.
Composites
A composite material is a material in which one or more mutually insoluble materials are mixed or bonded together. The primary classes of composites are particulate composites, fibrous composites, and laminated composites.
Particulate Composites
Particulate composites are created by adding particles of one material to a matrix (the filler material). The particles will typically account for less than 15% of the total material volume. The particles are added to improve upon some shortcoming of the matrix material.
Fibrous Composites
A fibrous composite is a material in which fibers of one material are embedded within a matrix. The fibers carry most of the stress, and the matrix serves to hold the fibers in place and to transmit stress between the fibers. The fibers can be short and randomly oriented, or they can be long and continuous.
Laminated Composites
Laminated composites are created by combining layers of composite materials. The layers will typically differ in the orientation of the fibers, or they will differ in the material itself. Sandwich materials are common, in which a lightweight material (such as foam or a honeycomb) will be placed in between layers of a strong, stiff material.
PDH Classroom offers a continuing education course based on this engineering materials reference page. This course can be used to fulfill PDH credit requirements for maintaining your PE license.
Now that you've read this reference page, earn credit for it!
Notes
Note 1: Material Property Data
The material property data provided are intended to be representative of the material described. The provided values tend toward the conservative end of the spectrum and could be used as baseline design values for preliminary design. However, these values do not conform to any particular specification, and so they should not be used in final design without first consulting with the appropriate material specifications. The data are provided "as is" without warranty of any kind, either expressed or implied. MechaniCalc, Inc. shall not be liable for any damages arising from the use of this data.
References
- Callister, William D., "Materials Science and Engineering: An Introduction," 9th Edition
- Dowling, Norman E., "Mechanical Behavior of Materials: Engineering Methods for Deformation, Fracture, and Fatigue," 4th Edition
- Lindeburg, Michael R., "Mechanical Engineering Reference Manual for the PE Exam," 13th Ed.
- Machinery's Handbook, 30th Edition, Industrial Press Inc.
- MMPDS-04, "Metallic Materials Properties Development and Standardization (MMPDS)," April 2008